Sleep Apnea Device Recall Drags on, Stoking Frustration
WASHINGTON — A significant recall of millions of snooze apnea machines has stoked anger and annoyance between clients, and U.S. officers are weighing unprecedented legal motion to speed a substitution exertion that is established to drag into following year.
Sound-dampening foam in the pressurized respiration devices can split down about time, foremost buyers to perhaps inhale little black particles or hazardous chemical compounds while they snooze, producer Philips warned in June 2021.
Philips initially estimated it could restore or exchange the models in a yr. But with the recall growing to more than 5 million gadgets throughout the world, the Dutch company now suggests the energy will extend into 2023.
That is left many clients to opt for involving working with a possibly destructive device or seeking risky therapies, including eradicating the foam on their own, acquiring next-hand equipment online or only heading without having the remedy.
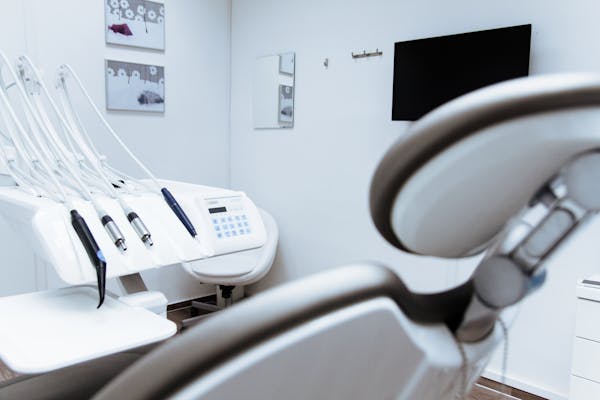
The devices are identified as continuous good airway pressure, or CPAP, machines. They force air by means of a mask to maintain passageways open throughout rest.
Read Extra: Is Loud night breathing Unsafe? Here’s When to Be concerned
Untreated sleep apnea can cause persons to prevent breathing hundreds of situations for each evening, main to dangerous drowsiness and increased coronary heart assault hazard. The trouble is far more frequent in men than females, with estimates ranging from 10% to 30% of older people influenced.
Most clients are better off utilizing a recalled machine because the threats of untreated snooze apnea still outweigh the opportunity harms of the disintegrating foam, medical professionals say. But medical practitioners have been hard pressed to assist clients obtain new equipment, which generally price between $500 and $1,000, and ended up already in short source due to provide chain problems.
“What transpired is the business just said, ‘Talk to your doctor.’ But medical practitioners just cannot manufacture new machines out of the blue,” said Dr. John Saito, a respiratory expert around Los Angeles.
Hazards from the foam incorporate headache, asthma, allergic reactions and most cancers-leading to results on internal organs, according to the Foods and Drug Administration. The recalled products contain Dreamstation and SystemOne CPAP designs and various other Philips equipment, like Trilogy ventilators.
Past March, the Food and drug administration took the scarce action of ordering Philips to grow its interaction effort, including “clearer details about the well being dangers of its products and solutions.” Regulators approximated then that only half of U.S. people afflicted had registered with the firm.
The company hadn’t issued this sort of an get in many years.
Read through Far more: Narcan Can Help save an Opioid User’s Life. What to Know About the Drug
In a assertion, Philips claimed ongoing tests on the recalled products is “encouraging” and exhibits lower amounts of particles and chemical byproducts emitted by its major brand name of equipment. Philips reported its original communication about the dangers posed by the foam was “a worst-scenario situation for the feasible well being risks.” The deterioration seems to worsen with unauthorized cleansing methods, the corporation observed.
The Fda has received much more than 70,000 reviews of troubles attributed to the equipment, which includes pneumonia, an infection, headache and cancer. These types of experiences are not independently confirmed and just cannot prove a causal relationship. They can be submitted by companies, individuals, physicians or lawyers.
Jeffrey Reed, of Marysville, Ohio, experienced been employing his Philips equipment for about a year when he began viewing black specks in the tubing and mask. His products supplier mentioned the particles was induced by inappropriate cleaning, so he ongoing working with it.
Over the next 7 yrs, Reed claims he knowledgeable persistent sinus infections, such as two bouts of pneumonia, that did not solve with antibiotics. Just after hearing about the recall, he suspected the foam particles might be actively playing a purpose.
“Once I bought off their device, all of that cleared ideal up,” mentioned Reed, 62, who attained a competitor’s gadget after quite a few months. Like other consumers, Reed cannot definitively confirm his troubles were being triggered by Philips’ unit.
More than 340 private harm lawsuits from Philips have been consolidated in a Pennsylvania federal courtroom and hundreds much more are anticipated in coming months. Reed is not portion of the litigation.
Like the wide greater part of U.S. CPAP customers, Reed got his machine by a clinical equipment provider contracted by his insurer. The corporation went out of business right before the recall and he by no means listened to from them about a substitution.
Even in standard circumstances, people corporations ordinarily really don’t track sufferers lengthy phrase.
Go through A lot more: For Young children with Very long COVID, Good Treatment Is Hard to Locate
“After a pair years, you’re just forgotten in the process,” said Ismael Cordero, a biomedical engineer and CPAP user. “I stopped hearing from my provider about a few several years just after I got my machine.”
Cordero acquired that his Philips equipment experienced been recalled by means of his get the job done at ECRI, a nonprofit that opinions professional medical system protection.
In Could, the Food and drug administration place Philips on detect that it was considering a second purchase that would pressure the organization to enhance and speed up its repair-and-switch method.
Professional medical system companies generally carry out recollects voluntarily, and previous Food and drug administration officials say the agency has under no circumstances truly utilised its authority to power more methods.
“The Fda shares the frustrations expressed by clients who are awaiting a resolution for this remember,” the agency explained in a assertion. Philips even now hasn’t supplied “all info we requested to consider the dangers from the chemical substances launched from the foam.”
Philips disclosed earlier this year that it received a Office of Justice subpoena around the recall. The company has not publicly commented on the subject, for each federal policies.
But an Fda inspection of Philips’ Pennsylvania workplaces uncovered a spate of purple flags final drop, which include e-mails suggesting the enterprise was warned of the dilemma 6 years ahead of the recall. In an Oct 2015 email, one particular customer appeared to alert Philips that the polyester polyurethane foam could degrade, according to Fda.
Amongst 2016 and early 2021, Food and drug administration identified 14 cases wherever Philips was made informed of the issue or was examining the trouble internally. “No even further layout improve, corrective motion or discipline correction was performed,” the Food and drug administration inspectors consistently notice.
In a May possibly 2018 email, foam supplier William T. Burnett wrote to Philips in an electronic mail: “We would not recommend use of polyester foam in this sort of an surroundings. … It will ultimately decompose to a sticky powder,” in accordance to an affidavit filed as part of a lawsuit above the foam.
Considering that the recall, Philips has been using a new style of foam manufactured from silicone to refurbish machines.
But Fda alerted individuals very last November that the new content had unsuccessful just one basic safety examination. And regulators questioned the organization to accomplish additional testing to make clear any health pitfalls with the two the new foam and the recalled content. Philips suggests impartial screening has not identified any safety troubles.
The enterprise suggests it has replaced or fixed about 69% of recalled products globally and aims to ship 90% of those people asked for by year’s finish. On common, the company produces about 1 million rest products per year.
“We have scaled up by additional than a variable of 3, but inevitably it nevertheless will take time to remediate 5.5 million products globally,” the organization claimed. About 50 percent are in the U.S.
Jeffrey Reed is among those nonetheless waiting around.
Reed registered for a substitute product in June 2021 — inside a 7 days of the remember. This thirty day period, he gained an e-mail from Philips indicating that his machine has been discontinued and is not available for quick substitute. In its place, the organization offered him $50 to return the equipment or an possibility of providing additional info to get a more recent a person.
“For them to hold out until eventually October to notify me that my equipment is far too outdated, when they’ve identified specifically what product I have since the working day I registered — which is aggravating,” Reed reported. “It’s disappointing that a supplier of lifestyle-preserving equipment treats people today like this.”
Far more Should-Read Tales From TIME